Tim Cook hints at new health products beyond the Watch; talks Apple TV, iPad Pro killing PCs, encryption


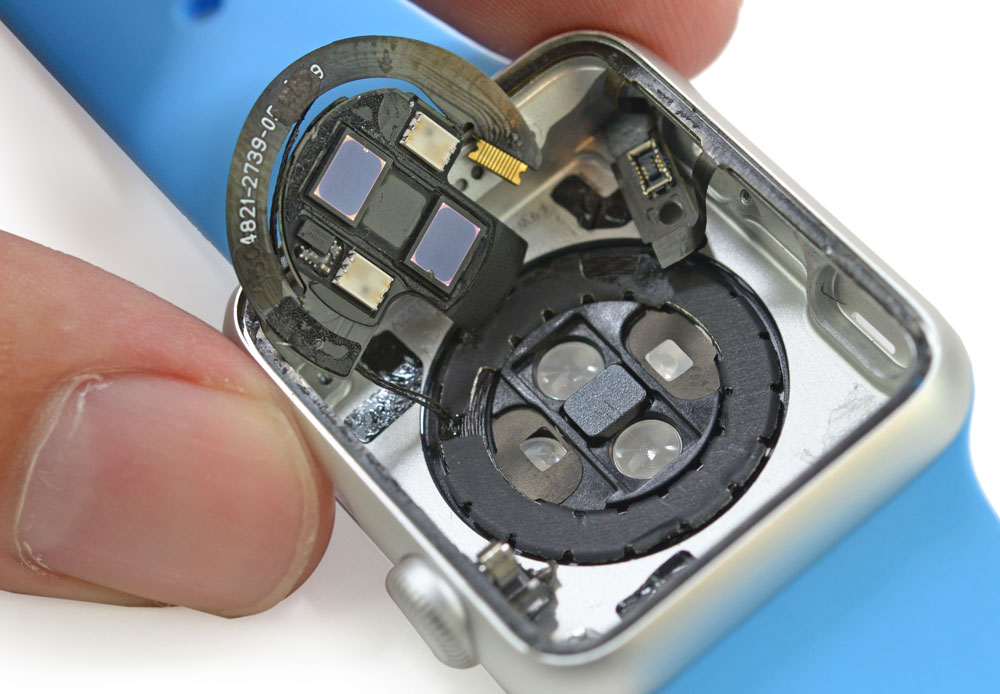
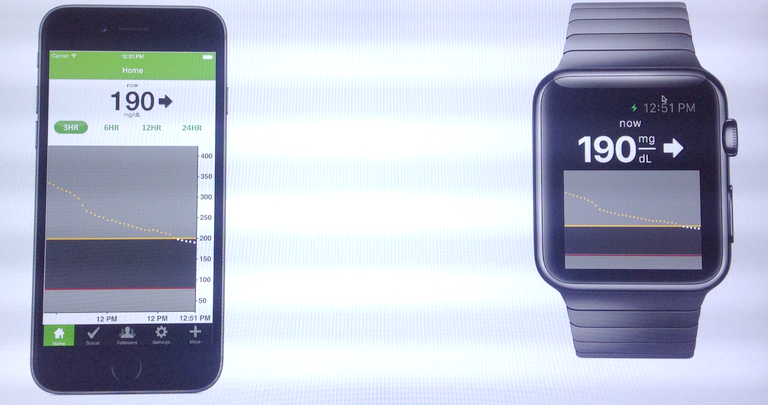
In a wide-ranging interview with the Telegraph, Apple CEO Tim Cook has hinted that the company may launch more health-focused products in future – but will keep those separate from the Apple Watch. The reason, he says, is that the FDA approval needed for full-on health devices would slow down the pace of innovation of the Watch.
Cook hints that Apple may have more plans for the health sphere, in a revelation which will intrigue Wall Street, but he doesn’t want the watch itself to become a regulated, government-licensed health product. “We don’t want to put the watch through the Food and Drug Administration (FDA) process. I wouldn’t mind putting something adjacent to the watch through it, but not the watch, because it would hold us back from innovating too much, the cycles are too long. But you can begin to envision other things that might be adjacent to it — maybe an app, maybe something else.”
This represents a significant change from expectations …


 Following Apple employees’
Following Apple employees’ 


