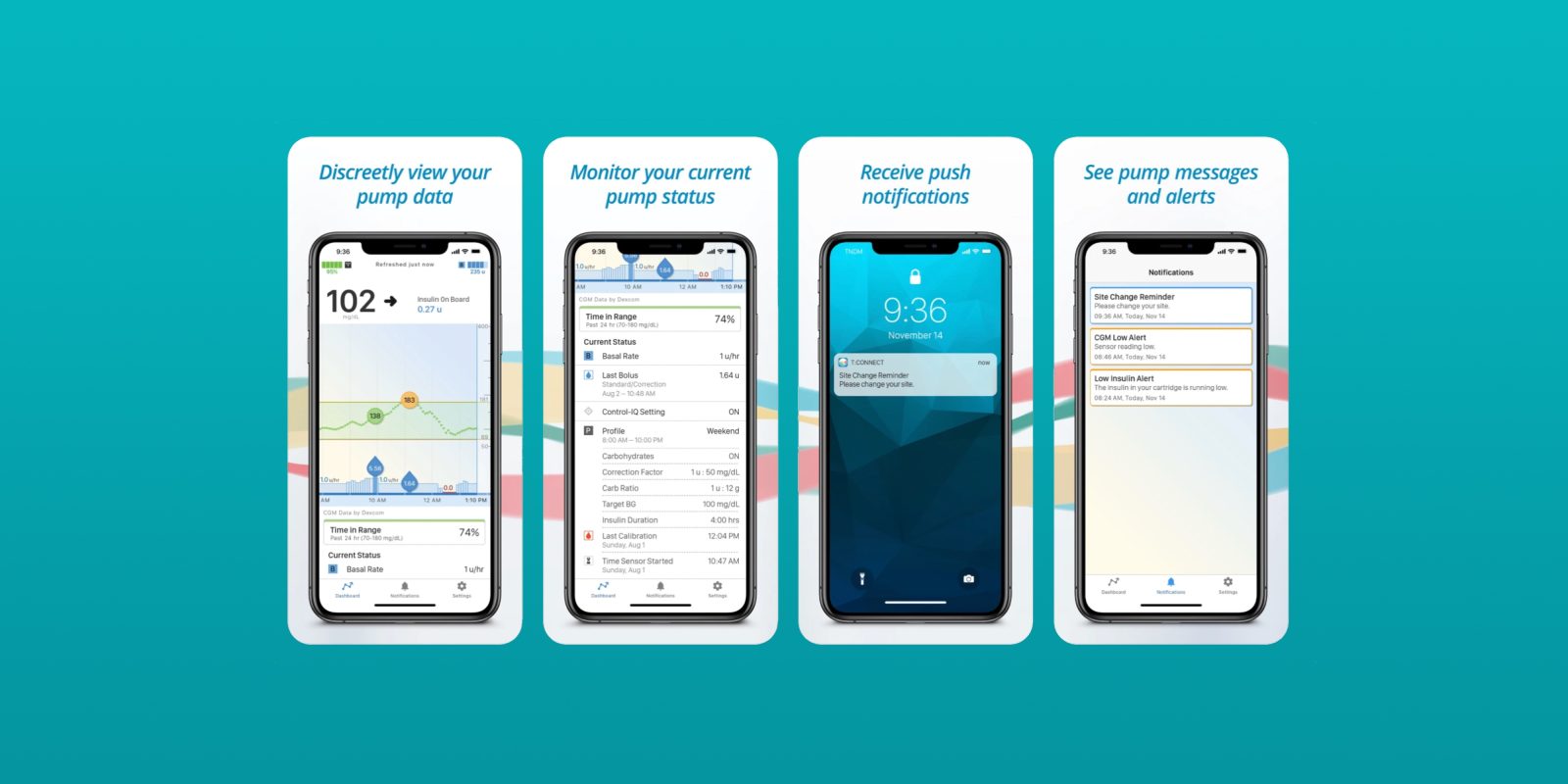
The Food and Drug Administration has cleared what is described as the first-ever smartphone application capable of initiating insulin delivery on both iOS and Android operating systems. The system here comes from Tandem Diabetes Care, and the application ties in to the company’s t:slim X2 insulin pump.
Prior to this approval from the FDA, delivery of insulin had to be handed through the pump itself. This change, however, means that users will be able to “program or cancel bolus doses of insulin” using the app for iPhone and Android devices, according to The Verge. Previously, the app could be used for monitoring trends and historical data, but not for initiating the delivery of insulin.
“This FDA clearance further validates our commitment to innovation and the diabetes community by providing one of the most requested feature enhancements,” said John Sheridan, president and CEO of Tandem Diabetes Care. “With the improvements in diabetes management provided by Tandem’s Control-IQ technology, giving a meal bolus is now the most common reason a person interacts with their pump, and the ability to do so using a smartphone app offers a convenient and discrete solution.”
Tandem Diabetes Care says that it will make this feature available to existing users at no additional cost. The feature will begin rolling out this spring with a series of “limited launch groups,” with a full launch later in the summer.
When released, this new feature will be offered in the United States for no additional cost to new t:slim X2 insulin pump customers and to in-warranty customers through a remote software update for the t:slim X2 insulin pump and the updated t:connect mobile app. The Company intends to roll out the mobile bolus feature update throughout the spring in a series of limited launch groups, followed by an expanded launch later this summer. Limited launch participants have already been selected.
This approval from the Food and Drug Administration comes as Apple itself is said to be investigating potential diabetes treatment technology for the Apple Watch. While Apple’s roadmap in this area is ambitious, more recent reports have said that the company has hit multiple roadblocks. One report in 2017 indicated that Tim Cook had been seen actively wearing a glucose tracker prototype on his body around Apple’s campus.
You can learn more about today’s announcement in Tandem Diabetes Care’s full press release right here.
FTC: We use income earning auto affiliate links. More.



Comments